Sustainability in Packaging: Migration, Testing, and Regulation
Release time:
2025-06-25
I. Migration Phenomena and Food Packaging Risks
Definition: Chemical transfer from printed layers to packaged food due to molecular size (typically <1000 Da) and chemical properties.
High-Risk Packaging Types Requiring LM Inks:
Packaging Format | Risks without LM Inks | Key Mechanism |
1. Pouches for Dry Food | - Reel-to-reel production causes set-off risk - Aluminum barrier layer inhibits diffusion - Degradation products penetrate PE layer | Pressure transfer in multilayer structures |
2. In-Mold/Self-Adhesive Labels on Cups | - Stack storage induces set-off - Cup thickness (>200μm) reduces diffusion risk | Static pressure |
3. Shrink Sleeves (PP/PE/PS/PET) | - Diffusion risk: Low to medium - Direct migration risk proportional to bottle storage time/wall thickness | Material permeability variance |
II. Three-Dimensional Migration Mechanisms
Set-off Migration
Trigger: Direct contact between printed layer and food-contact surface under reel pressure (>0.1 MPa) or stack load (>50 kg/m²).
Process: Physical transfer of uncured monomers/photoinitiators under pressure (Image 1).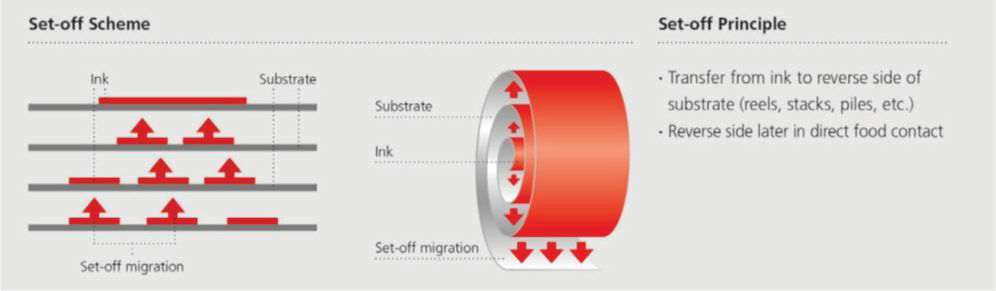
Image 1,Set-off migration can occur between a surface printed layer to a non-printed food contact surface when in close contact due to the pressure existing in the reel or stack
Diffusion Migration
Two-stage Path:
Stage 1: Pre-filling period; small molecules (MW<500 Da) penetrate substrate inward.
Stage 2: Post-filling; lipophilic substances (e.g., mineral oils) extracted by food (Image 2).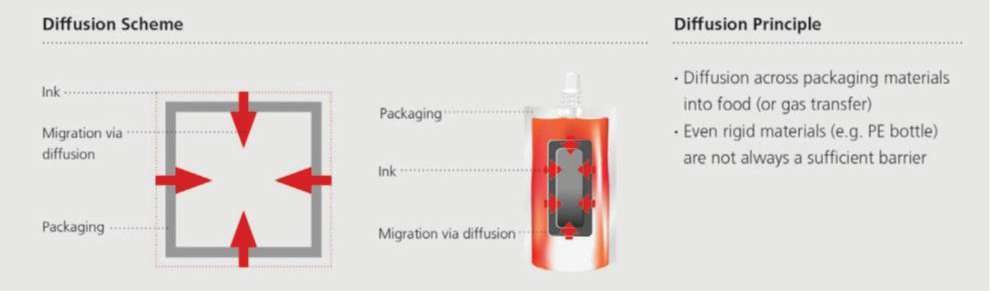
Image 2,Diffusion migration can occur due to the ease with which small and mobile molecules can penetrate across packaging material layers.
Gas Phase Migration
Typical Migrants: Mineral oils (MOSH/MOAH), semi-volatile UV photoinitiators (e.g., BP, ITX).
Unique Path: Volatilization from substrate → adsorption onto food via headspace (Image 3).

Image 3,Migrants,such as mineral oils or some UV photoinitiators,can migrate from the substrate via the gas phase.
III. Core Design Principles of Low Migration (LM) Ink Systems
Molecular Weight Threshold Principle:
Empirical rule: Components with MW > 1000 Da exhibit negligible migration (diffusion coefficient ∝ 1/√MW).
LM Formulation Components:
High-MW photoinitiators (e.g., acylphosphine oxides, MW 800-1200 Da)
Oligomers (acrylate-based, MW 1500-5000 Da)
Monomers (high-functionality to reduce residues)
Vs. Standard Inks: Standard inks contain low-MW substances (200-500 Da) with high migration risk (Image 4).
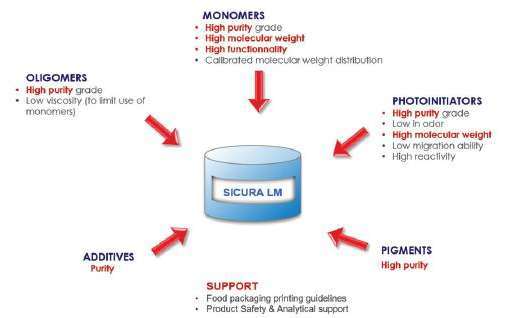
Image 4,Formulation components of low-migration inks
IV. Global Food Contact Material Regulations
Europe/Middle East/Africa (EMEA)
Regulation | Core Requirements | Technical Criteria |
(EC) No 1935/2004 | - Article 3: No health hazard, no unacceptable food composition/organoleptic changes | General safety framework |
(EC) No 2023/2006 (GMP) | - No direct food contact with printed surface - Migration/invisible set-off below limits | QMS certification |
(EU) No 10/2011 (Plastics) | - Plastic materials only - Positive list (Union List) - Overall Migration Limit (OML)=60 mg/kg food - Specific Migration Limits (SMLs) (e.g., BPA=0.6 ppm) | Migration test: 10 days/40°C for fatty simulants |
Swiss Ordinance (817.023.21)
World’s first positive list for printing inks:
List A: Toxicologically assessed substances (SMLs/OMLs, e.g., aniline SML=0.01 ppm)
List B: Non-assessed substances (detection limit ≤10 ppb)
Scope: Intentionally Added Substances (IAS) & non-direct food contact (non-DFC) inks only
German Ink Ordinance (21st Consumer Goods Amendment)
Transition period: Full implementation by January 1, 2026
Rules for unlisted substances:
Prohibited for DFC applications
Non-CMR (carcinogenic/mutagenic/reprotoxic) classification required
Migration must be <10 ppb
Non-Intentionally Added Substances (NIAS): Self-assessment per international scientific principles (e.g., EFSA guidelines)
Key Americas & Asia Regulations
Region | Regulation | Technical Highlights |
USA | FDA 21 CFR 175.300 (indirect additives) | "Threshold of Regulation" (TOR) compliance (≤0.5 ppb dietary exposure) |
China | GB 4806.1-2016 (general safety) GB 9685-2016 (additives) | - Chapter A4 lists ink additives (e.g., titanium dioxide ≤1%) - Toxicological data submission required |
Southeast Asia | Indonesia Reg.20/2019 Vietnam Law 55/2010 Philippines RA 10611 | EU framework reference; partial adoption of OML=60 mg/kg |
MERCOSUR | GMC RES. 03/92 (framework) RES.56/92 (plastics) RES.02/12 (details) | Migration limits aligned with EU |
V. Comprehensive Migration Testing Protocol
1. Test Basis & Simulant Selection
Regulatory reference: (EU) No 10/2011 Annex III
Food simulant matrix:
Food Type | Simulant | Test Conditions |
Aqueous (pH>4.5) | 10% ethanol | 10 days/40°C |
Acidic (pH≤4.5) | 3% acetic acid | 10 days/40°C |
Fatty | 50% ethanol or isooctane | 10 days/40°C (ethanol) 2 days/20°C (isooctane) |
2. Worst-Case Scenario Sampling Strategy
Critical parameter coverage:
Sample handling protocol:
Industrial storage simulation: Stack pressure ≥100 kg/m², 40±2°C, 60% RH
Transport protection: Aluminum foil wrapping (prevent contaminant adsorption)
Traceability: Sample ID linked to ink batch/curing energy/storage duratio
3.Testing Methods (Images 8-9)


Image 8(above),9(below),Printed samples cam be exposed to simulant using a standard cell or directly filled in the packaging.
Direct filling: For sealed containers (e.g., PEHD bottle sleeves, PES cups)
Steps: Simulant injection → sealing → incubation → HPLC/GC-MS analysis
Standard cell exposure: For flat materials (e.g., labels)
Equipment: EU migration cell (1-2 dm² area)
Conditions: Simulant contact with single printed layer (60±5 μm thickness)
4. Data Conversion & Compliance Thresholds
Unit conversion formula:
- Migration(μg/kg)=Detected value(μg/dm2)×6 dm21 kg foodMigration(μg/kg)=Detected value(μg/dm2)×1kgfood6dm2
Compliance limits:
OML: ≤60 mg/kg
SML: e.g., BPA ≤0.6 mg/kg
Unlisted substances: ≤10 ppb (0.01 mg/kg)
5. Industry Guidance Supplement
EuPIA Migration Test Guidance (www.eupia.org) key recommendations:
NIAS screening: GC×GC-TOFMS non-targeted analysis
Photoinitiator degradation products: Mandatory assessment (e.g., benzophenone photolysis products)

Reference:
Brown, C. (2023). Sustainability in Packaging: Migration, Testing and Regulation. UV+EB Technology, Q4 2023, 42-45.
Regulatory Sources:
(EU) No 10/2011: https://eur-lex.europa.eu
Swiss Ordinance 817.023.21: https://www.admin.ch
German Ink Ordinance: https://www.bvl.bund.de
food packaging
Previous Page
Latest News
Get a Free Consultancy
NANTONG EASTO MATERIALS TECHNOLOGY CO.,LTD.

No.118,Zhujiang Rd.,Juegang St.,Rudong County,
Nantong City,Jiangsu Province,226400,China




 2025-06-25
2025-06-25