Hydrophilicity and Hydrophobicity: From Molecules to UV/EB Curing
Release time:
2025-08-17
A drop of water rolls off a lotus leaf like a pearl, while it quickly spreads and wets a glass surface. This common phenomenon in daily life is a result of the "intimate conversation" between a material's surface and water molecules: hydrophilicity and hydrophobicity. Understanding these "twin" properties is key to unlocking everything from cell membrane function to the design of high-tech coatings.
1) Definitions
Hydrophilicity: This refers to the tendency of a material or chemical group to have a favorable interaction with water. Hydrophilic surfaces attract water, causing a water drop to spread (low contact angle). Hydrophilic molecules tend to dissolve or swell in water.
Hydrophobicity: This refers to the tendency of a material or chemical group to avoid water. Hydrophobic surfaces repel water, causing a water drop to form a bead (high contact angle). Hydrophobic molecules have poor solubility in water.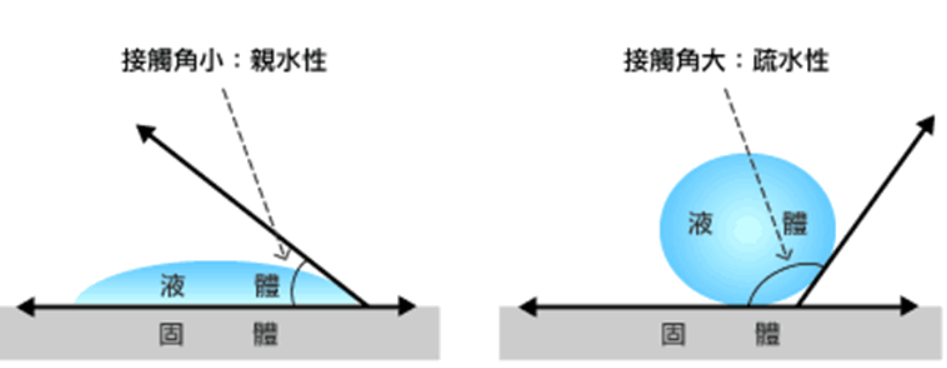
Figure 1: A conceptual diagram shows a water drop on two flat surfaces. On the left, the water drop spreads with a contact angle of approximately 20° (hydrophilic). On the right, the water drop forms a bead with a contact angle of approximately 110° (hydrophobic).
2) Mechanism of Action
Hydrogen Bonds and Polarity: Water is highly polar and can form hydrogen bonds. Molecules with hydrogen bond donors (such as -OH, -NH) or acceptors (such as C=O, ether O) interact strongly with water and exhibit hydrophilicity.
Hydrophobic Effect: Non-polar surfaces disrupt the hydrogen bond network of water. Water minimizes contact by clustering non-polar parts together, which macroscopically manifests as the formation of a water bead.
Interfacial Energy Balance: Wetting is governed by Young's equation: γSV = γSL + γLVcosθ. A lower solid-liquid interfacial tension (γSL) or lower liquid surface tension promotes spreading (a smaller contact angle θ).
Morphology/Roughness: Micro- and nano-scale roughness can amplify a material's inherent wetting properties. For example, a rough hydrophilic surface can become superhydrophilic. A rough hydrophobic surface (combined with low surface energy chemicals) can become superhydrophobic (Cassie–Baxter state).
3) Structure-Property Relationship
Hydrophilicity/hydrophobicity are not binary attributes but a continuous spectrum that can be adjusted through functional groups, chain length/branching, and copolymer composition. In a cross-linked network, the distribution of segments at the surface (migration during the curing process) largely determines the measured contact angle.
Structural Feature | Typical Examples | Effect on Water Interaction | Considerations for Formulators |
Polar Carbonyl, Ether, Hydroxyl Groups | –C(=O)–O– (ester), –OH, –O– | Increases dipole/hydrogen bonding → more hydrophilic | Increases water absorption; improves adhesion to polar substrates (glass, metal) |
Ionic Groups | Carboxylates, Quaternary Ammonium Salts | Strongly absorb water → very hydrophilic | Can cause swelling; useful in water-based/dispersion systems |
Long Alkyl Chains | C8–C18 side chains | Large non-polar regions → more hydrophobic | Enhances water resistance, lubricity; may reduce adhesion to polar surfaces |
Fluorinated/Siloxane Segments | –CF₂–, –CF₃; –Si–O– | Ultra-low surface energy → strong hydrophobic/oleophobic | Excellent release/stain resistance; risk of migration and interlayer adhesion issues |
Hydroxylated Acrylates | HEMA, HEA | Hydrophilic reactive diluents | Improves pigment wetting/adhesion; increases viscosity and water absorption |
Bulky/Aromatic Cores | Styrenes, Bisphenol Epoxies | Moderately polar + rigid | Balances wetting and hardness; generally good adhesion to glass after using coupling agents |
4) (Meth)acrylates: Hydrophilicity/Hydrophobicity Control and Applications in UV/EB Curing
(Meth)acrylate monomers (e.g., acrylic acid AA and methyl methacrylate MMA) are core raw materials for UV/EB Curing technology. By selecting different ester side chains (R), the hydrophilicity/hydrophobicity of the monomer can be precisely controlled, thereby imparting desired performance to the final cured coating.
General Structural Formula of (Meth)acrylate Monomers: CH₂=CR¹-COOR²
R¹ = H (acrylate) or CH₃ (methacrylate)
R² = Ester side chain (key for controlling hydrophilicity/hydrophobicity!)
Effect of Ester Side Chain (R²) on Hydrophilicity/Hydrophobicity:
Ester Side Chain (R²) Type | Representative Monomer | Hydrophilicity/Hydrophobicity | Primary Effect | Typical Application Scenarios (UV/EB Curing) |
Short-chain Alkyl/Hydroxylated Alkyl | Hydroxyethyl methacrylate (HEMA) | Strongly Hydrophilic | High surface energy, easily wets substrates | Water-based coatings, inks; adhesives; biocompatible coatings |
Medium-length Alkyl | Ethyl acrylate (EA) | Moderate | Balances flexibility and wettability | General-purpose coatings, adhesives |
| Butyl acrylate (BA) |
|
|
|
| Methyl methacrylate (MMA) | Slightly Hydrophobic | High hardness, good weather resistance | Hard coatings, plastic coatings |
Long-chain Alkyl | Lauryl acrylate (LA) | Hydrophobic | Lowers surface energy, increases hydrophobicity | Waterproof coatings; anti-fouling coatings; low-migration formulations |
| Stearyl acrylate (SA) |
|
|
|
Fluorinated/Silane Alkyl | Trifluoroethyl acrylate | Superhydrophobic/Oleophobic | Extremely low surface energy, excellent water/oil repellency | High-end anti-graffiti coatings; self-cleaning surfaces; superhydrophobic coatings |
| Silane acrylate |
|
|
|
Aromatic Ring | Phenyl acrylate | Hydrophobic | Increases hardness, rigidity, heat resistance | High-temperature coatings; high-hardness topcoats |
(Table Note: This table summarizes the effects of different ester side chains on the hydrophilicity/hydrophobicity of (meth)acrylate monomers and their typical applications in UV/EB curing.)
Application Strategies in UV/EB Curing Formulation Design:
Adjusting Wettability and Adhesion:
For substrates that are difficult to wet (e.g., polyolefin plastics PP/PE), adding a small amount of a strongly hydrophilic monomer (such as acrylic acid AA or HEMA) can significantly improve the formulation's wetting ability and is key to enhancing coating adhesion.
For high-energy surfaces like metal and glass, it is necessary to avoid excessive shrinkage stress that can lead to poor adhesion, and hydrophilic monomers also help to improve this.
Controlling Surface Properties (Waterproofing/Stain Resistance/Self-Cleaning):
When hydrophobic or superhydrophobic surfaces are needed (e.g., waterproofing outdoor wood paints, anti-fouling for building facades), the formulation should contain a large amount of long-chain alkyl monomers (like LA, SA) or fluorinated/silicone monomers. They migrate to the coating surface to lower its surface energy.
Synergistic Effect: Long-chain monomers are often used in combination with additives that promote surface enrichment (such as silicone leveling agents, fluorocarbon surfactants) to optimize the hydrophobic effect.
Affecting Coating Uniformity and Compatibility:
Excessive differences in the hydrophilicity/hydrophobicity of monomers in a formulation can lead to compatibility issues (haziness, phase separation). Monomers with similar solubility parameters (SP values) or suitable solvents/dispersion media must be carefully selected (this is especially important in water-based UV systems).
Water-based UV Curing: Hydrophilic monomers (e.g., HEMA, AA) are crucial for maintaining the stable dispersion of the resin in water. After curing, hydrophobic monomers (such as long-chain esters) contribute to water resistance.
Controlling Curing Kinetics and Film Properties:
Hydrophilic monomers (especially hydroxyl-containing HEMA) may participate in hydrogen bonding, slightly affecting free radical activity, sometimes requiring adjustments to the photoinitiator system.
Hydrophobic long-chain monomers typically provide better segment mobility, increasing coating flexibility, while hydrophilic or rigid monomers (like MMA) provide hardness.
Conclusion
Hydrophilicity and hydrophobicity, these seemingly simple properties, are in fact the microscopic language of the molecular world's "dialogue" with water molecules, with their secrets hidden in molecular structure and interfacial forces. From the lotus leaf's "untouched by mud," to the delicate separation of cell membranes, and to the precise tailoring of coating performance in modern (meth)acrylate-based UV/EB curing technology, the understanding and control of hydrophilicity and hydrophobicity have always been a core bridge connecting fundamental science with cutting-edge applications. In the future, with deeper research into super-wetting interfaces, biomimetic materials, and intelligent responsive surfaces, this "secret language" between water and matter will continue to lead a new wave of innovation in materials science.
Previous Page
Previous Page
Latest News
Get a Free Consultancy
NANTONG EASTO MATERIALS TECHNOLOGY CO.,LTD.

No.118,Zhujiang Rd.,Juegang St.,Rudong County,
Nantong City,Jiangsu Province,226400,China




 2025-08-17
2025-08-17







