Application and Development of UV-Curable Adhesives in Lithium-Ion Batteries(Part 2:The Intrinsic Connection Between Polymer Structure and Performance Optimization)
Release time:
2025-07-12
Introduction: Bridging the Gap—Exploring the Root Causes of Performance Differences
In Part I, we systematically evaluated the macroscopic performance of UV-curable adhesives in lithium-ion batteries, confirming their enormous potential as alternatives to traditional PVDF systems. However, one core question remains unanswered: Why do UV-curable adhesives exhibit higher charge transfer resistance (Rct) in EIS tests compared to traditional systems?
This seemingly unfavorable microscopic characteristic stands in sharp contrast to their excellent macroscopic performance. In this section, we build on the previous discussion and dive deep into the microscopic world of materials. Starting from the unique polymerization mechanism of UV curing, we will uncover the scientific essence behind this phenomenon and explore how fine-tuning polymer structures can optimize the ultimate performance of batteries.
Decoding the Mystery of High Impedance: The “Relative Heterogeneity” and Nanogel of UV Adhesives
To understand the puzzle of high internal resistance in UV-curable adhesives, we must first grasp their unique curing process. Unlike PVDF, which relies on solvent evaporation and physical entanglement of polymer chains, UV curing is a rapid chemical crosslinking process. Particularly when high-functionality monomers (such as dendritic acrylates) are involved, the photoinitiated free-radical polymerization occurs at extremely high speed, locally forming highly crosslinked, nano-sized polymer networks—known as nanogels.
These nanogel particles act as rigid “islands,” dispersed within a relatively linear and more flexible polymer “sea” formed by low-functionality monomers. This “island-sea” structure leads to the adhesive system exhibiting “relative heterogeneity” at the microscopic level.
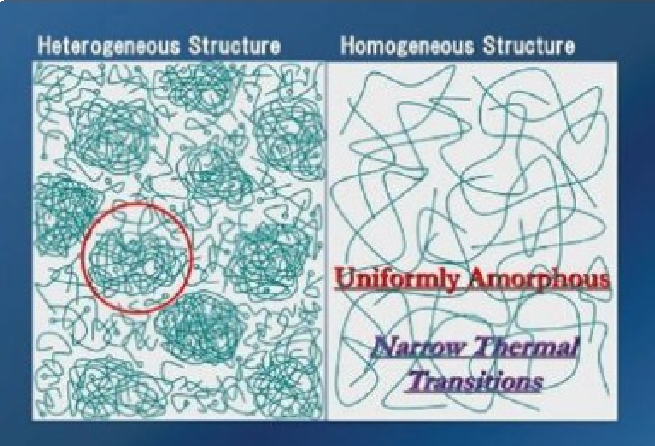
Diagram: Concept Illustration of Nanogels—Highly crosslinked nano-regions formed during UV curing create microstructural heterogeneity. (Source: UV+EB Technology, 2025 Q2, Figure 3)
This heterogeneous structure is the fundamental reason for the increased charge transfer resistance (Rct):
Spatial Hindrance: The dense nanogel regions act as physical barriers, making the lithium-ion migration paths within the electrode more tortuous, thus impeding transport.
Ion-Dipole Interactions: Nanogels are rich in polar groups (such as ester or ether groups), which can interact strongly with positively charged lithium ions. These ion-dipole interactions act like temporary “traps,” reducing the mobility of lithium ions at the electrode/electrolyte interface and increasing Rct.
Thus, the high Rct in UV-curable adhesives is not a defect but an inherent consequence of their unique polymerization mechanism.
Dynamic Mechanical Analysis (DMA): A Window into the Microstructure of UV Adhesives
Dynamic Mechanical Analysis (DMA) provides a powerful tool to validate the theories of nanogels and relative heterogeneity in UV-curable adhesives. By measuring how materials respond to periodic stress, DMA reveals their viscoelastic behavior—particularly their glass transition temperature (Tg).
For a homogeneous polymer, the Tan Delta curve (the ratio of loss modulus to storage modulus) typically exhibits a relatively sharp peak corresponding to a clear and narrow Tg. However, for UV-curable adhesives with relative heterogeneity, the DMA spectra display markedly different characteristics.
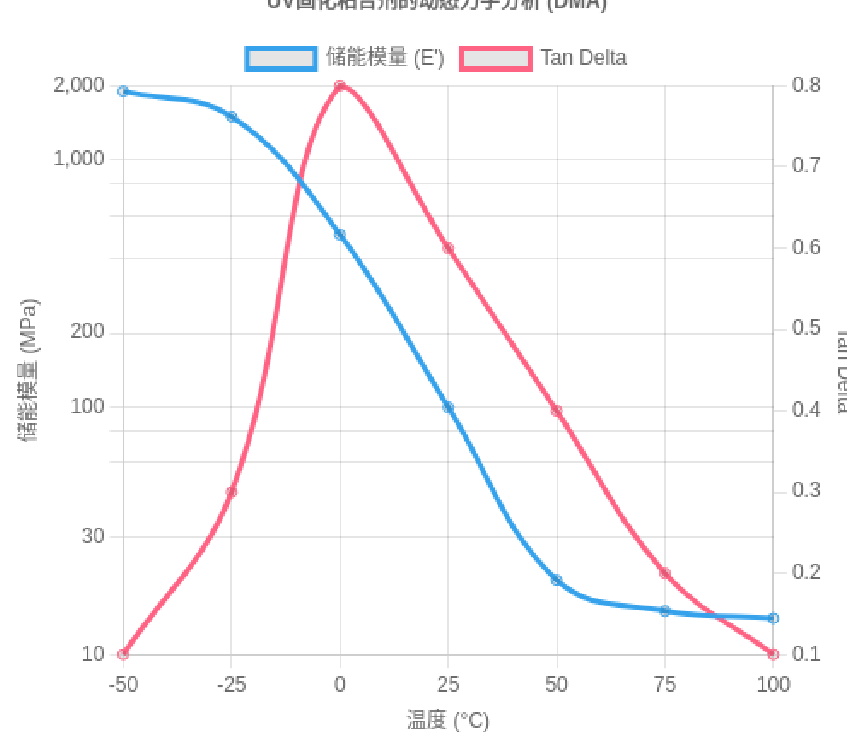
Diagram: DMA Spectrum of UV-Curable Adhesive—A broad Tan Delta peak spanning nearly 100°C directly demonstrates the microstructural heterogeneity. (Data source: UV+EB Technology, 2025 Q2, Figure 2)
The DMA results above clearly show that the Tan Delta peak of UV-curable adhesives is extremely broad, spanning nearly 100°C. This is direct evidence of the existence of multiple micro-regions with varying chain mobility within the system:
Some regions exhibit high crosslinking density and restricted motion (higher Tg), corresponding to the nanogel domains.
Other regions show lower crosslinking density and greater chain flexibility (lower Tg), corresponding to the polymer matrix.
This broad Tg distribution is a hallmark of the system’s relative heterogeneity.
Further comparisons between different formulations (for example, increasing the content of highly functional dendritic monomers) reveal that the Tan Delta peak broadens and the storage modulus rises significantly. This reinforces the intrinsic relationship between formulation design, microstructure, and mechanical performance.
From Microstructure to Macroscopic Performance: The Trade-Off Between Mechanical Properties and Battery Rate Performance
We can now return to the original question: How can an adhesive with higher internal resistance (Rct) still support a high-performance battery?
The answer lies in a classic case of performance trade-off.
The heterogeneous microstructure introduced by nanogels, while somewhat compromising ionic transport speed (resulting in higher Rct), dramatically enhances the mechanical properties of the adhesive. These highly crosslinked nanogel domains act like the “gravel” in reinforced concrete, significantly boosting the modulus, hardness, and dimensional stability of the adhesive network.
This leads to several critical macroscopic advantages:
Superior Structural Integrity:
The enhanced mechanical strength allows the electrode to better maintain its structure during the repeated volume expansion and contraction of lithium-ion insertion/extraction. This helps suppress the pulverization and detachment of active material particles.
Stable Interfaces:
Low swelling and high modulus properties ensure the long-term stability of the electrode/electrolyte interface, preventing the deterioration of interfacial resistance caused by adhesive swelling.
Thus, this is a deliberate trade-off:
The UV-curable adhesive sacrifices part of its ionic conductivity at the microscopic level to gain mechanical robustness at the macroscopic level. In battery operation—especially under conditions of long-term cycling or high-rate charging/discharging—this structural stability translates into significant performance benefits, such as lower capacity fade over time.
This explains the observation from Part I: despite higher Rct, UV-curable adhesives deliver superior cycle life and excellent overall electrochemical performance.
It highlights the complex, multi-factor nature of battery performance, which is jointly determined by electrochemical, mechanical, and thermal properties.
Key Takeaways from Part II
The higher charge transfer resistance (Rct) of UV-curable adhesives originates from their unique “relative heterogeneity” microstructure, specifically the formation of nanogels during UV curing.
The dense nanogel regions create:
Spatial hindrance, making lithium-ion migration paths more tortuous.
Ion–dipole interactions, temporarily trapping lithium ions and slowing down their migration across the electrode/electrolyte interface.
DMA testing reveals a broad Tan Delta peak, which is strong evidence of this heterogeneity—indicating the presence of diverse polymer microenvironments with varying mobility.
The performance of UV-curable adhesives represents a deliberate trade-off: sacrificing some ionic transport efficiency to gain outstanding mechanical strength and structural stability, which in turn supports better long-term battery reliability and cycling performance.
Conclusion: UV-Curing Technology for the Next Generation of High-Performance Batteries
Through the in-depth analysis in both parts of this study, we have gained a comprehensive and profound understanding of the application of UV-curable adhesives in lithium-ion batteries. These adhesives not only revolutionize battery manufacturing through improved production efficiency and environmental friendliness, but also demonstrate unique and competitive electrochemical and mechanical performance.
The key to the successful application of UV-curable adhesives lies in fully understanding and mastering their distinctive nanogel-based microstructure. This microstructure is simultaneously the source of their higher internal resistance and the foundation of their exceptional mechanical stability.
This recognition provides clear guidance for future research and development:
The focus should no longer be on chasing single extreme performance parameters (such as lowest possible resistance), but on systematic optimization—balancing mechanical, electrochemical, and processing performance.
By precisely tuning UV curing formulations (such as the chemical structure of monomers/oligomers, functionality ratios, photoinitiator systems, etc.), researchers can proactively design nanogel size, distribution, and crosslink density.
The ultimate goal is to maintain the mechanical advantages of UV adhesives while maximizing ion transport channels and minimizing internal resistance, thus achieving comprehensive optimization in:Energy density,Power density,Cycle life,Safety.
Undoubtedly, as research deepens and technology matures, UV-curing technology will play an increasingly important role in driving the next generation of high-performance, long-life, and high-safety lithium-ion batteries.
*Data source: UV+EB Technology, 2025 Q1/Q2
Previous Page
Latest News
Get a Free Consultancy
NANTONG EASTO MATERIALS TECHNOLOGY CO.,LTD.

No.118,Zhujiang Rd.,Juegang St.,Rudong County,
Nantong City,Jiangsu Province,226400,China




 2025-07-12
2025-07-12






