Understanding the “Outdoor Durability of Coatings” (Part 1): Core Mechanisms and Advanced Improvement Strategies
Release time:
2025-08-24
The outdoor durability of a coating, also known as weatherability, is its ability to maintain its protective and decorative functions in a complex and variable outdoor environment (such as sunlight, temperature changes, rain, and pollutants). This performance directly determines the aesthetic value and service life of assets such as building facades, car bodies, and bridge steel structures. Its failure not only affects the appearance but may also lead to substrate corrosion, resulting in huge economic losses. This article will provide an in-depth analysis of the root causes of coating degradation and systematically introduce the industry's advanced strategies for improving durability.
The Culprits of Coating Degradation: An Analysis of Environmental Stresses
The aging of coatings outdoors is not caused by a single factor, but rather by the synergistic effect of multiple stresses such as light, water, heat, and chemical substances.
Photo-induced Oxidative Degradation
Ultraviolet (UV) radiation in sunlight is the primary culprit of coating aging. According to the first law of photochemistry, only light absorbed by a substance can initiate a chemical reaction. When the polymer binder in a coating absorbs UV photons, it transitions to a high-energy excited state, which then causes chemical bonds to break and generates highly reactive free radicals. These free radicals react rapidly with oxygen in the air, initiating a chain auto-oxidation cycle that produces unstable hydroperoxides (POOH). Under the action of light and heat, POOH easily decomposes to generate more free radicals, forming an "avalanche-like" autocatalytic degradation, which ultimately leads to the scission of polymer chains and a sharp deterioration of the coating's macroscopic properties.
Therefore, in formulation design, the fundamental approach is to select resins that do not easily absorb UV light and have stable chemical bonds. For example, high-performance outdoor coatings often use aliphatic polyurethanes, while avoiding aromatic polyurethanes, which easily absorb UV light and yellow quickly.
Hydrolysis and Chemical Erosion
Water is another critical factor in coating degradation. On one hand, water molecules can penetrate the coating and hydrolytically attack functional groups such as ester groups and carbamates, destroying the polymer backbone or cross-linked network. On the other hand, water, as a solvent, dissolves acidic pollutants in the atmosphere (such as SO₂ and NOx) to form acid rain, which exacerbates the hydrolysis process and directly affects the coating's resistance to environmental corrosion. Studies have shown that polyurethane cross-linking systems generally have better acid resistance than melamine-formaldehyde systems. Furthermore, there is a significant synergistic effect between photo-oxidation and hydrolysis: hydrophilic groups such as carboxyl and hydroxyl groups generated by photo-oxidation increase the water absorption of the coating, thereby creating more favorable conditions for hydrolysis reactions.
The Way of Defense: Advanced Coating Stabilization Strategies
In the face of complex environmental challenges, modern coatings science has developed a "three-dimensional defense" system, from fundamental resin selection to the synergistic use of sophisticated additives, to comprehensively improve the outdoor durability of coatings.
Strategy 1: Genetic Selection of Resin Systems
Selecting highly weather-resistant resins is the first line of defense in creating long-lasting coatings. The chemical structure of different resins determines their inherent stability. For example, fluorocarbon resins (such as PVDF) show excellent weatherability due to the high bond energy of their C-F bonds. Organosilicon-modified polymers are favored for the stability of their Si-O bonds. In polyester systems, using neopentyl glycol (NPG) instead of other diols can utilize the steric hindrance effect of its tertiary carbon atoms to protect ester groups from hydrolytic attack, thereby significantly improving durability.
Strategy 2: “Sunscreen + Repair”—The Synergistic Action of Light Stabilizers
The addition of light stabilizers is a core technology for resisting photo-oxidation. The most effective strategy at present is the synergistic use of Ultraviolet Absorbers (UVA) and Hindered Amine Light Stabilizers (HALS):
Ultraviolet Absorber (UVA): Acting like the coating's "sunscreen," it can efficiently absorb harmful UV radiation and convert it into harmless thermal energy for release, thereby protecting the polymer binder. It is especially important for protecting the underlying layer of the coating and the substrate.
Hindered Amine Light Stabilizer (HALS): It plays the role of a "free radical scavenger" and "repairer". HALS and its derivatives can efficiently capture and clear various free radicals generated during degradation, interrupting the auto-oxidation chain reaction. Its unique feature is that it can be regenerated during the reaction, forming a catalytic cycle, thus providing long-term protection at very low concentrations.
The combination of UVA and HALS achieves a complementary effect of "source blocking" and "process scavenging," and is widely recognized by the industry as the best light stabilization solution.
Strategy 3: The Double-Edged Sword Effect and Optimization of Pigments
The impact of pigments on durability cannot be ignored. Some pigments, such as high-quality carbon black, are excellent UV shields in their own right. However, some pigments can also act as "catalysts" for degradation. The most typical example is titanium dioxide (TiO₂), the most commonly used white pigment, whose anatase crystal form has strong photocatalytic activity, generating highly oxidizing free radicals under UV irradiation, which severely damages the resin binder and leads to "chalking". To solve this problem, modern processes involve dense inorganic (such as silicon oxide and aluminum oxide) and organic surface coatings on highly weather-resistant rutile-type TiO₂ to effectively inactivate its photocatalytic activity, making it an ideal pigment with both excellent hiding power and high durability.
Case Study: How Nanotechnology Reshapes Coating Durability

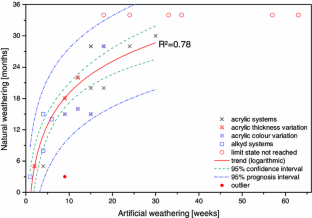
The chart compares the abrasion resistance of coatings with different types of nanofillers added to that of unmodified coatings.
Next Page
Latest News
Get a Free Consultancy
NANTONG EASTO MATERIALS TECHNOLOGY CO.,LTD.

No.118,Zhujiang Rd.,Juegang St.,Rudong County,
Nantong City,Jiangsu Province,226400,China




 2025-08-24
2025-08-24






